Hospital Instructions for Influenza Surveillance and Reporting, 2019-2020 Season
Influenza-associated hospitalizations and influenza-associated deaths are reportable to the Connecticut Department of Public Health (DPH). This information is shared with relevant local health departments through the CT Electronic Disease Surveillance System (CTEDSS).
The Emerging Infections Program (EIP) at the Yale School of Public Health conducts enhanced surveillance activities for residents of Middlesex and New Haven Counties on behalf of the DPH and in this capacity is acting as an agent of the State. These enhanced surveillance data contribute to FluSurv-NET, CDC’s Influenza Hospitalization Surveillance Network, which covers over 70 counties in 13 states. FluSurv-NET data are used to estimate age-specific hospitalization rates and describe characteristics of persons hospitalized with severe influenza illness. Staff of the DPH or Yale EIP may request supplemental information on patients. If you have questions, please contact Alan Siniscalchi (DPH: 860-509-7994) or Kim Yousey-Hindes (Yale: 203-764-5942).
Reporting is available through the web-based CTEDSS and we encourage you to use this option as it will help speed dissemination of information. Reporting is also done by faxing the completed Hospitalized and Fatal Cases of Influenza case report form, which is available on the DPH Forms web page, to the DPH Epidemiology and Emerging Infections Program (EEIP) at 860-509-7910. For information about how hospital staff can acquire access to CTEDSS, contact the CTEDSS team at dph.ctedss@ct.gov.
Influenza-associated Hospitalizations
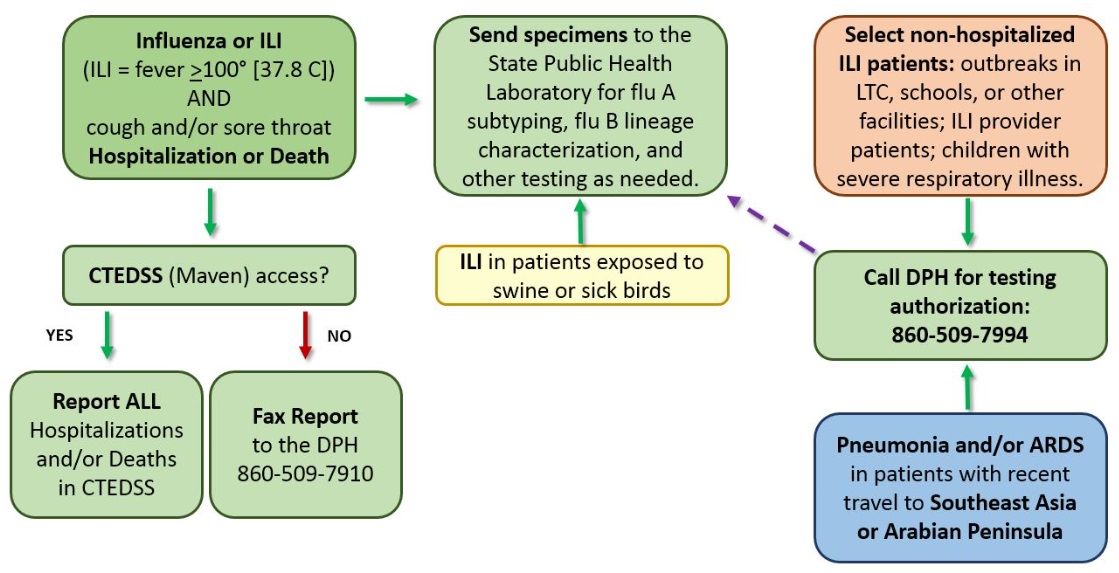
Must be reported within 12 hours on the day of recognition or strong suspicion of possible influenza infection (i.e. patients with compatible illness regardless of the results of the initial rapid antigen and/or DFA test). Respiratory specimens from all flu-associated hospitalizations should be submitted to the CT DPH State Public Health Laboratory (SPHL) for PCR testing.
Influenza-associated Deaths
All possible influenza-associated deaths must be reported to the DPH within 12 hours, even if influenza was not the primary cause of death. Save respiratory specimens and submit them to the SPHL for post-mortem PCR testing. Reporting is conducted by web-based entry into CTEDSS or by faxing case report forms to the DPH EEIP at 860-509-7910. Provide both date and causes of death. For after hours or holiday reporting, report on the next normal business day.
Criteria for Submission of Specimens to the SPHL for PCR Testing
To monitor circulating influenza strains throughout the season and to determine the effectiveness of this season’s vaccines, subtyping is done on specimens obtained from the following patients who present with influenza-like illness (ILI: fever ≥100°F [37.8°C] plus cough and/or sore throat), regardless of rapid antigen testing status.
- All hospitalized patients with ILI or confirmed influenza
- Selected non-hospitalized patients with ILI including: a) patients of ILI network (ILINet) providers, b) patients associated with outbreaks in long-term care, schools, or other facilities, and c) in children, severe respiratory illness with or without fever. Please contact the DPH EEIP at 860-509-7994 to discuss testing of 2a-c, including possible respiratory viral panel (RVP) testing for enterovirus and other respiratory viruses.
- All patients with ILI and recent close exposure to swine, sick poultry at farms and agricultural settings, or migratory birds (exposure history should be provided);
- All patients with pneumonia and/or Acute Respiratory Distress Syndrome (ARDS) developing within 17 days of travel to Southeast Asia or within 14 days of travel in or near the Arabian Peninsula, contact the DPH EEIP at 860-509-7994 to discuss possible avian flu or Middle East Respiratory Syndrome Coronavirus [MERS-CoV] testing. Travel history should be provided.
Healthcare providers must follow the testing instructions and complete the Laboratory Clinical Test Requisition form (OL-9B) to submit specimens for PCR testing. There is no charge for this service.
Connecticut Influenza Surveillance: Summary of Special Instructions for Hospitals
This page last updated 10/08/2019.

